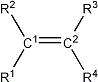
The SCS values used for the calculations are taken from [1,2]. Both papers contain a much finer division of functional groups, e.g. different SCS values are given for conjugated and non-conjugated compounds. However, an exhaustive list would become unmanageable so only a subset is used here. In most cases the loss of accuracy is not great.
Since values for the 1H increments of fluoro, nitro and sulfoxides were not given in [1] they were estimated using published NMR data.
Literature
[1] "Estimation of the chemical shifts of olefinic protons using additive increments - II The compilation of additive increments for 43 functional groups"Mater UE, Pascual C, Pretsch E, Pross A, Simon W, Sternhell S Tetrahedron 25 (1969) 691-697
[2] "Parameter set for the prediction of the 13C-NMR chemical shifts of sp2- and sp-hybridized carbon atoms in organic compounds"
Pretsch E, Fürst A, Robien W Anal. Chim. Acta 248 (1991) 415-428
|
